Congratulations to Connor Thompson, a PhD student in Professor François Jean's lab, for winning the Antibody Society's 2025 Science Writing Competition! Read his entry here:
A Different Kind of Magic Bullet:
The Case for Host-Directed Antibody Therapies as Antivirals
By Connor Thompson
“We must search for magic bullets. We must strike the parasites, and the parasites only, if possible, and to do this, we must learn to aim with chemical substances!”
— Paul Ehrlich, 1908 Nobel Laureate in Physiology or Medicine1
Imagine a future where a single medicine can stop multiple pandemics before they begin – not by chasing every new virus, but by outsmarting how they use our own biology against us. Broad-spectrum, host-directed antibodies might make this future possible. The above quote captures Ehrlich’s pioneering concept of the “magic bullet”, a therapeutic agent that specifically targets disease-causing organisms without harming the host. While this idea laid the foundation for modern chemotherapy and has had a lasting impact on medical sciences, it overlooks a key emerging concept in modern virology, that as well as targeting viruses directly we can also disable them by targeting host pathways that viruses depend upon.
In the race against viral pandemics, science must not only catch up to emerging threats but stay ahead. The COVID-19 pandemic exposed critical vulnerabilities in our antiviral arsenal, including how quickly viruses can evolve to outmaneuver therapies designed to treat them. Hopes were initially high for monoclonal antibodies like bamlanivimad and casirivimab developed for treating SARS-CoV-2, but faltered as the virus rapidly evolved and became resistant 2,3. When new viral variants such as Delta and Omicron rendered many treatments less effective, it became clear that we need better antiviral tools that are not only powerful but also resilient. One of the most promising avenues for future antiviral development lies in a deceptively simple idea: don’t just target the virus – target us 4. Specifically, target the common human (host) factors that diverse viruses exploit. This strategy, know as host-directed therapy, may be our best hope for broad-spectrum antiviral protection. Among host-directed antivirals, monoclonal antibodies (mAbs) stand out for their specificity and modifiability. In the future, a single shelf-stable, inhaled antibody spray targeting host receptors could be stockpiled, for instance in airports and hospitals, to halt a novel respiratory virus before it spreads.
What are host-directed monoclonal antibodies?
Most antiviral antibodies are designed to target viral proteins, such as the spike protein of SARS-CoV-2. These are often effective, until the virus mutates. Unlike viruses, human host proteins evolve much more slowly. Viruses depend on these relatively stable host factors to infect and replicate inside cells, making them attractive therapeutic targets. Host-directed monoclonal antibodies bind to specific human proteins involved in the viral life cycle, such as receptors the virus uses to enter cells or signaling molecules that modulate immune responses. Because these therapies act on the host rather than the virus, they are less likely to lose effectiveness when new viral variants arise. Novel emerging viruses may also depend upon these shared host pathways, potentially allowing for effective antivirals to be in place for a virus that doesn’t even exist yet!
Why target the host instead of the virus?
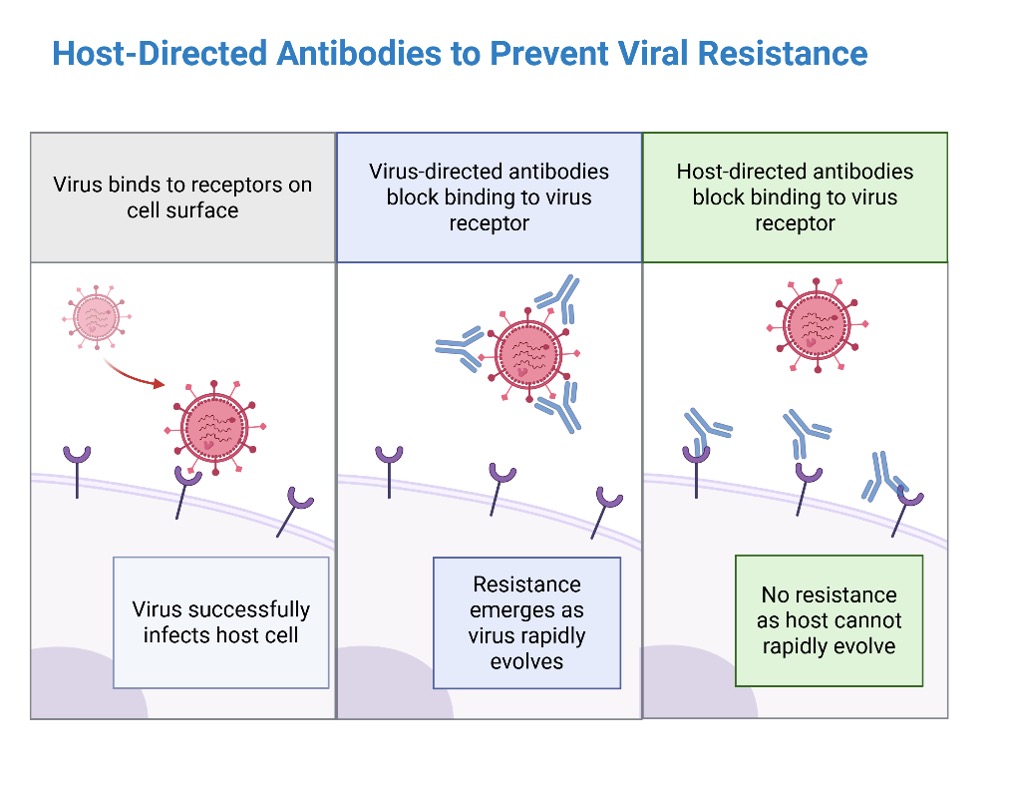
There are several compelling reasons to invest in host-targeted monoclonal antibodies. The most straightforward is reducing the risk of viral resistance. Since host-directed antibodies target human proteins rather than viral proteins, these antibodies are less vulnerable to viral mutations and escape variants. Many different viruses hijack the same host pathways, so targeting those shared host components could yield cross-viral protection and broad-spectrum antiviral activity. Target conservation is another consideration as human proteins change slowly over time, offering a more stable and reliable target for therapeutic intervention. One advantage that viruses have over us is their rapid capacity to evolve, by targeting them indirectly through host-directed therapies we can limit this. To see how this works in practice, in the next section let’s look at how host-directed antibodies could be used to block viral entry into human cells.
Blocking viral entry points
One of the most obvious uses of host-targeted antibodies is to block viral entry into cells. By binding to receptors or coreceptors on human cells, these antibodies can prevent viruses from attaching, entering, and replicating. This has already been demonstrated to be possible for treating human disease, for example the monoclonal antibodies semzuvolimab and ibalizumab bind to the CD4 receptor on T cells, preventing human immunodeficiency virus 1 (HIV-1) from entering its target cells 5,6. Other examples are the use of anti-claudin-1 and anti-occludin antibodies to block tight-junction proteins used by hepatitis C virus (HCV) to infect liver cells 7,8. Crucially, these therapies still remain effective even if the virus itself mutates. By blocking viral entry into host cells, we can block infection.
Additional host targets besides viral entry
Host-directed monoclonal antibodies offer other therapeutic benefits beyond blocking viral entry. Some, like anti-CD69 antibodies, enhance the immune response by increasing immune cell migration and cytokine production, boosting both innate and adaptive antiviral defenses 9. Others mitigate virus-induced tissue damage, as seen with bevacizumab, which reduces scarring and neovascularization in HSV-1-infected corneas by inhibiting VEGF 10. Additionally, host-directed mAbs can synergize with traditional antivirals; for example, combining anti-CD3 therapy with interferon and ribavirin improves viral control in chronic HCV by enhancing regulatory T cell responses 11. Together, these approaches highlight the multidimensional potential of host-targeted antibodies for both acute and chronic viral infections. Of course, targeting host pathways isn’t risk-free, it can potentially introduce side effects or immune complications. But with precise engineering and targeted delivery, these risks can be minimized, especially compared to the threat of viral resistance.
Emerging technologies in host-directed antibody design
New technologies are accelerating the development of host-directed monoclonal antibodies with broader and more effective antiviral capabilities. AI and machine learning can aid in predicting conserved epitopes and designing cross-reactive antibodies, while nanobodies and single-domain antibodies offer compact, stable formats ideal for targeting membrane-bound host proteins 12. Phage display libraries enable rapid screening of billions of candidates for broad-spectrum activity 13. We can harness evolutionary approaches to generate new effective antibodies to keep up with viral threats. Beyond mAbs alone, combining them with other host-targeted or direct-acting (viral-targeted) therapies could enhance antiviral effects and reduce the risk of resistance, offering a powerful, adaptable strategy against both current and emerging viral threats. This in turn would provide even more protection against the possible emergence of resistance.
Preparing for future pandemics
The COVID-19 pandemic was a stark reminder of how new viral threats can rapidly emerge and overwhelm our healthcare systems. Climate change, deforestation, and increased global travel are accelerating the spread of zoonotic viruses (pathogens that jump from animals to humans). Many of these pathogens, such as Ebola, influenza, Nipah virus, or novel coronaviruses, could spark future pandemics. Broad-spectrum monoclonal antibodies, especially those targeting shared host factors, offer a powerful platform for pandemic preparedness. By focusing on human components that are common to multiple viruses, we can build therapies that are effectively virus-agnostic and variant-proof. Unlike virus-specific vaccines or drugs that must be reformulated for each strain, host-directed antibodies offer the potential of universal protection—especially valuable for lower-resource regions during future pandemics. The economic and logistical advantages are clear, we could avoid the slow and costly vaccine approval process, the need for periodic re-vaccination, as well as the contentious social issues related to vaccine mandates and public compliance.
Conclusion: Developing powerful new antibody-based therapies for pandemic preparedness
In an age where viruses evolve quickly and new pathogens can emerge at any moment, it is clear that we need better and smarter antiviral strategies. Host-directed monoclonal antibodies offer a compelling alternative by targeting the more stable biology of human cells rather than constantly mutating viruses. These therapies can potentially work across multiple viruses, avoid resistance, boost the immune response, and pair well with other treatments. Thanks to advances in antibody engineering and rapid screening technologies, they’re also becoming faster to develop. If we invest now (both scientifically and collaboratively) these broad-spectrum antibodies could become a cornerstone of how we prevent and respond to future viral outbreaks. This isn't just a new tool—it's a new paradigm: instead of reacting to viruses, we pre-empt them by targeting their dependencies on us. By turning our biology into a barrier rather than a vulnerability, we may finally build a future where viral pandemics become preventable history.
References
1. Dale, H. H. The Collected Papers of Paul Ehrlich (ed. Himmelweit, F.) 1–18 (Pergamon, 2013). doi:10.1016/B978-0-08-009054-2.50004-3.
2. Choudhary, M. C. et al. Emergence of SARS-CoV-2 escape mutations during Bamlanivimab therapy in a phase II randomized clinical trial. Nat. Microbiol. 7, 1906–1917 (2022).
3. Focosi, D., McConnell, S., Sullivan, D. J. & Casadevall, A. Analysis of SARS-CoV-2 mutations associated with resistance to therapeutic monoclonal antibodies that emerge after treatment. Drug Resist. Updat. Rev. Comment. Antimicrob. Anticancer Chemother. 71, 100991 (2023).
4. Host-Directed Antiviral Therapy | Clinical Microbiology Reviews. https://journals.asm.org/doi/10.1128/cmr.00168-19.
5. Wang, C.-Y. et al. Effect of Anti-CD4 Antibody UB-421 on HIV-1 Rebound after Treatment Interruption. N. Engl. J. Med. 380, 1535–1545 (2019).
6. Emu, B. et al. Phase 3 Study of Ibalizumab for Multidrug-Resistant HIV-1. N. Engl. J. Med. 379, 645–654 (2018).
7. Evans, M. J. et al. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature446, 801–805 (2007).
8. Shimizu, Y. et al. Monoclonal Antibodies against Occludin Completely Prevented Hepatitis C Virus Infection in a Mouse Model. J. Virol. 92, e02258-17 (2018).
9. Cibrián, D. & Sánchez-Madrid, F. CD69: from activation marker to metabolic gatekeeper. Eur. J. Immunol. 47, 946–953 (2017).
10. Sharma, S., Mulik, S., Kumar, N., Suryawanshi, A. & Rouse, B. T. An Anti-Inflammatory Role of VEGFR2/Src Kinase Inhibitor in Herpes Simplex Virus 1-Induced Immunopathology. J. Virol. 85, 5995–6007 (2011).
11. Halota, W. et al. Oral anti-CD3 immunotherapy for HCV-nonresponders is safe, promotes regulatory T cells and decreases viral load and liver enzyme levels: results of a phase-2a placebo-controlled trial. J. Viral Hepat. 22, 651–657 (2015).
12. Valdés-Tresanco, M. S., Valdés-Tresanco, M. E., Jiménez-Gutiérrez, D. E. & Moreno, E. Structural Modeling of Nanobodies: A Benchmark of State-of-the-Art Artificial Intelligence Programs. Molecules 28, 3991 (2023).
13. Alfaleh, M. A. et al. Phage Display Derived Monoclonal Antibodies: From Bench to Bedside. Front. Immunol. 11, (2020).